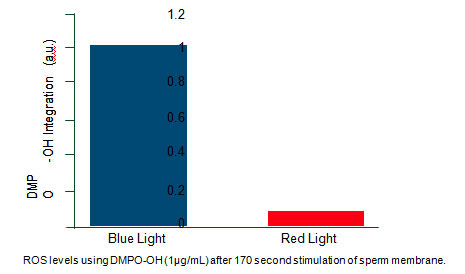
THE COMMITMENT TO RESEARCH
Since 1996, Erchonia, the manufacturer of Lunula, has been committed to fully elucidating the medical utility of low-level laser therapy through rigorous clinical studies. For over a decade, Erchonia has studied the clinical utility of low-level laser devices for the treatment of numerous medical ailments, and their recent device, Lunula, looks to revolutionise the way clear and healthy nails are restored following infection by fungus..
Lunula has been markedly studied – from the early in-vitro analysis to the extensive in-vivo studies – and its clinical utility to enable unsightly toenails to grow clear and healthy has been substantiated. The unique dual-diode approach of Lunula fortifies the body’s natural defense mechanisms against any infectious agent, and accelerates the growth of the nail. This multifaceted approach is the first of its kind, providing a truly effective, yet safe, way to enable the growth of strong, clear and healthy nails even when they may have previously been infected with fungal spores.
As you will quickly learn, Lunula is supported by an unwavering clinical foundation of both histological and clinical evidence that upholds the viability of this approach and ensures an effective procedure.